It may seem rather strange to have a project on spinning yarn in a chemistry class, but a great deal of the chemical industry was originally motivated by the textile industry. Synthetic polymers have a huge impact on modern textiles, but even in ancient times chemistry and textiles were intertwined. As we will see, cloth provided the motivation for the development of soap, dyes, bleach, and sulfuric acid.
Textiles decay much more quickly than artifacts made of stone or bone. Consequently there are very few surviving examples of the earliest textiles. However, the earliest cave art and in clay sculptures depict clothed human figures. From this and other indirect evidence it is believed that clothing made of spun fibers (as distinct from hides) goes back at least 20,000 years. The earlist style of clothing, the string skirt, was worn exclusively by women and consisted of a simple belt with strings hanging from the waist to above the knees.
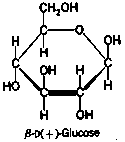
We begin with a discussion of the natural materials from which cloth is made. Prior to the advent of synthetics, there were two major divisions of the raw fibers: those from plant sources and those from animal sources. We are already familiar with the major component of all plant fibers: cellulose. We have been using the formula CH2O as the formula for cellulose, but this tells us nothing about the structure. Cellolose consists of long chains of carbon atoms with oxygen and hydrogen atoms sticking out from the chain. Plant cells are not able to reuse cellulose from the soil. The only compounds which can be used by the plant conisist of small molecules like carbon dioxide, water, and the soluble minerals dissolved in the water. Through the process of photosynthesis, carbon dioxide and water are combined into sugars, of which the most important is glucose: C6H12O6.
Glucose is a relatively small molecule with structure:

Pay particular attention to the OH groups sticking out all over it. These OH groups look kind of like half a water molecule and there is an old adage in chemistry "like dissolves like." This suggests that because of the OH groups, glucose will dissolve in water, a fact with which you are probably familiar.
A recurring theme in biochemistry is that big molecules are most easily built from small molecules. Plant cells are able to hook two glucose molecules together with the elimination of a water molecule:

This process, called polymerization, can be repeated over and over, the chain getting longer and longer, and the resulting chain is called cellulose.

The compicated structures we have drawn are called structural formulas and they give the details of how all the atoms are connected together. The formula C6H12O6 is an example of a molecular formula. It tells how many atoms of each kind are present in a molecule but it tells little about how those atoms are connected together. And looking at the molecular formula, we notice that there are twice as many hydrogens as carbons and the same number of oxygens as carbons. This gives us the empirical formula, CH2O, with which we are already familiar.
Fibers from animal sources are very different from plant fibers but at a deeper leverl there is a remarkable similarity in the way they are formed. Animal fibers, like wool and silk, are made from protein: long chains of smaller molecules called amino acids. Like cellulose, proteins are polymers of smaller molecules. In cellulose, the small molecules, or monomers, are all identical. In proteins, however, there are twenty three different amino acids which can be connected together in any order to form proteins with different structural and chemical properties.
Here is the simplest of the amino acids, glycine:
H H O N-C-C-OH H H
Its empirical formula is the same as its molecular formula, C2H5O2N.
Notice that it has an NH2 group at one end and an OH group at the other. Just as glucose monomers can be polymerized to form cellulose, amino acids can be polymerized to form proteins. Here, for example, is a polymer formed from glycine:
H O H O H O H O H O
...-N-C-C-N-C-C-N-C-C-N-C-C-N-C-C-...
H H H H H H H H H H
An enormous variety of proteins can be formed, depending on the sequence of amino acids in the chain. Hemoglobin is a protein. Meat and skin are composed largely of proteins. Hair and wool are also composed of proteins. The most important thing for us to remember for now is that both plants and animals build long molecules from much smaller ones.
The twine quiz consists of three questions on the following topics.
The earliest, and perhaps most important step on the road to modern textiles was the notion of taking short lengths of plant or animal fiber and combining these fibers into a much longer cord. Exactly how this was discovered will perhaps never be known. But by playing with a handful of fur or wool, it is not difficult to "reinvent" a simple procedure for spinning a cord using nothing but the raw fiber and two hands.
To begin with we need a fiber suitable for spinning. My own personal excursion into spinning were done with dog wool from my German Shepard, Sandy. I say dog "wool" rather than dog "hair" intentionally. For spinning we need a fiber that will cling to itself. Hair is slick and does not stick to itself readily. The wool that I harvested from Sandy came, not from her back or shoulders, but from her chest and armpits. Unlike the hair on her back, which is designed to shed water, this wool is soft and fluffy and clings to itself when twisted in the hands.
In earlier incarnations of this course I encouraged students to find fibers on their own. Many were inventive, choosing a wide variety of plant fibers and even human hair. For the most part, however, the fibers found were far inferior to dog wool and many students requested bits of Sandy for their spinning project. Sandy has passed away now and I am comforted by the many bits of her that remain in the form of string and cord. For the current course, I have located a local sheep farm, Serendipidy Farm, and I have an entire fleece (one sheep's worth) of wool for use in this class. This wool is unwashed and unprocessed, exactly as it came from the sheep.
To learn the basics of spinning, begin with a handful of wool. Pinch off a bit fiber and draw it out untill it separates from the rest of the wool. Examine these fibers, their length and elasticity, and put this pinch aside. Now draw off another pinch of wool but this time don't pull it so far that it separates from the rest of the wool. Now give it a little twist by twirling it between your fingers and draw some more wool from the handful. As you draw these new fibers out, you will find that the twist from the previous fibers will travel up the new section. By alternately twisting and drawing you can spin a piece of yarn a foot or more in length.
At this point what you really need is a third hand to hold and twist the yarn as one hand hold the wool and the other draws out the fibers. The drop spindle was invented to take the place of this third hand. Using the drop spindle we can spin yarn whose length is limited only by the amount of wool at our disposal. I will demonstrate the use of the drop spindle in class. We have drop spindles for class use which you can check out from me for a deposit of $5. Your deposit will be returned when you return the spindle. Please return the spindle as soon as possible so that everyone who wants to use one has access.
Checking out a drop spindle is not your only option. It is perfectly possible, though tedious, to spin using only your hands. You may also construct your own drop spindle using, for example, a stick for the spindle and an apple, potato, or ball of clay for the whorl.
If you allow your yarn to untwist, it will lose its strength and will come apart. If you use your yarn for weaving in a later project, the weaving itself will prevent the yarn from unraveling. If, on the other hand, the yarn is to be used as a cord, say, for fishing, stringing a trap, or making a net, the yarn can be plied or twisted back on itself. This can be accomplished by spinning one yarn clockwise, another counter-clockwise, and then spinning these two yarns together into a single cord. For short lengths, however, there is a shortcut. Simply add a lot of twist to the yarn, fold it in half, and let it twist back on itself. This technique will be demonstrated in class.
There is a remarkable similarity between the way cells build long molecules from small ones and the way in which we spin long yarns from short fibers.

Using your hands or a drop spindle, spin no fewer than 20 feet of yarn. Once you get the hang of it, you can spin a surprisingly large amount of yarn while involved in some other activity. I have spun 20 feet of yarn while watching an hour of television. You may leave your yarn as a single ply or you may produce 10 feet of 2-ply cord.
A drop spindle is not a complicated machine. Some cavemen have made them
from a pencil, a bar of soap, and a paperclip. I made a bunch of drop
spindles for use by the class. They can be had for a $10 deposit, returnable
when you return the spindle.
Click on the picture to get a slideshow of the
instructions.
When you have finished spinning your yarn and taking your quiz, turn them both in for evaluation. To pass, your yarn must be at least 20 feet long (or 10 feet for 2-ply) and you must miss no questions on the quiz.






